| Revision as of 22:51, 9 July 2019 editNeils51 (talk | contribs)Extended confirmed users114,962 editsm →Clinical significance: sp← Previous edit | Revision as of 19:08, 25 July 2019 edit undo141.209.57.178 (talk) Added etymology; removed redundant reference linksNext edit → | ||
| Line 2: | Line 2: | ||
| ] | ] | ||
| '''Vimentin''' is a structural ] that in humans is encoded by the ''VIM'' ]. Its name comes from the ] ''vimentum'' which refers to an array of flexible rods.<ref>{{cite journal |last1=Franke |first1=WW |last2=Schmid |first2=E |last3=Osborn |first3=M |last4=Weber |first4=K |title=Different intermediate-sized filaments distinguished by immunofluorescence microscopy. |journal=Proc Natl Acad Sci U S A |date=October 1978 |volume=75 |issue=10 |pages=5034-8 |pmc=336257}}</ref> | |||
| '''Vimentin''' is a structural ] that in humans is encoded by the ''VIM'' ]. | |||
| ] with antibody to reveal vimentin containing intermediate filaments in green and antibody to ] to reveal lysosomes in red. Nuclear DNA is seen in blue. Antibodies and image courtesy ].]] | ] with antibody to reveal vimentin containing intermediate filaments in green and antibody to ] to reveal lysosomes in red. Nuclear DNA is seen in blue. Antibodies and image courtesy ].]] | ||
| Vimentin is a type III ] (IF) protein that is expressed in ] cells. IF proteins are found in all ]<ref name="Eriksson">{{cite journal |vauthors=Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD |title=Introducing intermediate filaments: from discovery to disease |journal=J Clin Invest |year=2009 |volume=119 |issue=7 |pages=1763–71 |
Vimentin is a type III ] (IF) protein that is expressed in ] cells. IF proteins are found in all ]<ref name="Eriksson">{{cite journal |vauthors=Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD |title=Introducing intermediate filaments: from discovery to disease |journal=J Clin Invest |year=2009 |volume=119 |issue=7 |pages=1763–71 |pmc=2701876}}</ref> as well as ].<ref name="Cabeen">{{cite journal |vauthors=Cabeen MT, Jacobs-Wagner C |title=The bacterial cytoskeleton |journal=Annu Rev Genet |year=2010 |volume=44 |pages=365–92 |pmid=21047262}}</ref> IF, along with ]-based ] and ]-based microfilaments, comprises the ]. All IF proteins are expressed in a highly developmentally-regulated fashion; vimentin is the major cytoskeletal component of ] cells. Because of this, vimentin is often used as a marker of mesenchymally-derived cells or cells undergoing an ] (EMT) during both normal development and ] progression. | ||
| == Structure == | == Structure == | ||
| A vimentin monomer, like all other intermediate filaments, has a central α-helical ], capped on each end by non-] amino (head) and carboxyl (tail) domains.<ref name="fuchs">{{cite journal |author1=Fuchs E. |author2=Weber K. |title=Intermediate filaments: structure, dynamics, function, and disease |journal=Annu Rev Biochem |volume=63 |issue= |pages=345–82 |year=1994 |pmid=7979242 |
A vimentin monomer, like all other intermediate filaments, has a central α-helical ], capped on each end by non-] amino (head) and carboxyl (tail) domains.<ref name="fuchs">{{cite journal |author1=Fuchs E. |author2=Weber K. |title=Intermediate filaments: structure, dynamics, function, and disease |journal=Annu Rev Biochem |volume=63 |issue= |pages=345–82 |year=1994 |pmid=7979242}}</ref> Two monomers are likely co-translationally expressed in a way that facilitates their formation of a coiled-coil dimer, which is the basic subunit of vimentin assembly.<ref name="chang">{{cite journal |vauthors=Chang L, Shav-Tal Y, Trcek T, Singer RH, Goldman RD |title=Assembling an intermediate filament network by dynamic cotranslation |journal=J Cell Biol |volume=172 |issue=5 |pages=747–58 |year=2006 |pmc=2063706}}</ref> | ||
| The α-helical sequences contain a pattern of hydrophobic amino acids that contribute to forming a "hydrophobic seal" on the surface of the helix.<ref name="fuchs" /> In addition, there is a periodic distribution of acidic and basic ] that seems to play an important role in stabilizing ] dimers.<ref name="fuchs" /> The spacing of the charged residues is optimal for ionic ], which allows for the stabilization of the α-helix structure. While this type of stabilization is intuitive for intrachain interactions, rather than interchain interactions, scientists have proposed that perhaps the switch from intrachain salt bridges formed by acidic and basic residues to the interchain ionic associations contributes to the assembly of the filament.<ref name="fuchs" /> | The α-helical sequences contain a pattern of hydrophobic amino acids that contribute to forming a "hydrophobic seal" on the surface of the helix.<ref name="fuchs" /> In addition, there is a periodic distribution of acidic and basic ] that seems to play an important role in stabilizing ] dimers.<ref name="fuchs" /> The spacing of the charged residues is optimal for ionic ], which allows for the stabilization of the α-helix structure. While this type of stabilization is intuitive for intrachain interactions, rather than interchain interactions, scientists have proposed that perhaps the switch from intrachain salt bridges formed by acidic and basic residues to the interchain ionic associations contributes to the assembly of the filament.<ref name="fuchs" /> | ||
| Line 16: | Line 16: | ||
| == Function == | == Function == | ||
| Vimentin plays a significant role in supporting and anchoring the position of the organelles in the ]. Vimentin is attached to the ], ], and ], either laterally or terminally.<ref name="katsumoto">{{cite journal |author1=Katsumoto T. |author2=Mitsushima A. |author3=Kurimura T. |title=The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction |journal=Biol Cell |volume=68 |issue=2 |pages=139–46 |year=1990 |pmid=2192768 |
Vimentin plays a significant role in supporting and anchoring the position of the organelles in the ]. Vimentin is attached to the ], ], and ], either laterally or terminally.<ref name="katsumoto">{{cite journal |author1=Katsumoto T. |author2=Mitsushima A. |author3=Kurimura T. |title=The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction |journal=Biol Cell |volume=68 |issue=2 |pages=139–46 |year=1990 |pmid=2192768}}</ref> | ||
| The dynamic nature of vimentin is important when offering flexibility to the cell. Scientists found that vimentin provided cells with a resilience absent from the microtubule or actin filament networks, when under mechanical stress ''in vivo''. Therefore, in general, it is accepted that vimentin is the cytoskeletal component responsible for maintaining cell integrity. (It was found that cells without vimentin are extremely delicate when disturbed with a micropuncture).<ref name="goldman">{{cite journal |vauthors=Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM | title = The function of intermediate filaments in cell shape and cytoskeletal integrity | journal = J |
The dynamic nature of vimentin is important when offering flexibility to the cell. Scientists found that vimentin provided cells with a resilience absent from the microtubule or actin filament networks, when under mechanical stress ''in vivo''. Therefore, in general, it is accepted that vimentin is the cytoskeletal component responsible for maintaining cell integrity. (It was found that cells without vimentin are extremely delicate when disturbed with a micropuncture).<ref name="goldman">{{cite journal |vauthors=Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM | title = The function of intermediate filaments in cell shape and cytoskeletal integrity | journal = J Cell Biol. | volume = 134 | issue = 4 | pages = 971–83 |date=August 1996 |pmc = 2120965}}</ref> ] mice that lack vimentin appeared normal and did not show functional differences.<ref name="Golucci-Guyon">{{cite journal |vauthors=Golucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C |title=Mice lacking vimentin develop and reproduce without an obvious phenotype. |journal=Cell |year=1994 |volume=79 |issue=4 |pages=679–94 |pmid=7954832}}</ref> It is possible that the microtubule network may have compensated for the absence of the intermediate network. This result supports an intimate interactions between microtubules and vimentin. Moreover, when microtubule depolymerizers were present, vimentin reorganization occurred, once again implying a relationship between the two systems.<ref name="goldman" /> On the other hand, wounded mice that lack the vimentin gene heal slower than their wild type counterparts.<ref name="Eckes2000">{{cite journal |vauthors=Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P | title = Impaired wound healing in embryonic and adult mice lacking vimentin. | journal = J Cell Sci | year = 2000 | volume = 113 | pages = 2455–62 | pmid = 10852824}}</ref> | ||
| In essence, vimentin is responsible for maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions. Vimentin has been shown to eliminate toxic proteins in ] ] in asymmetric division of mammalian ].<ref name="pmid24843142">{{cite journal |vauthors=Ogrodnik M, Salmonowicz H, Brown R, Turkowska J, Sredniawa W, Pattabiraman S, Amen T, Abraham AC, Eichler N, Lyakhovetsky R, Kaganovich D | title=Dynamic JUNQ inclusion bodies are asymmetrically inherited in mammalian cell lines through the asymmetric partitioning of vimentin | journal=] | year=2014 | |
In essence, vimentin is responsible for maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions. Vimentin has been shown to eliminate toxic proteins in ] ] in asymmetric division of mammalian ].<ref name="pmid24843142">{{cite journal |vauthors=Ogrodnik M, Salmonowicz H, Brown R, Turkowska J, Sredniawa W, Pattabiraman S, Amen T, Abraham AC, Eichler N, Lyakhovetsky R, Kaganovich D | title=Dynamic JUNQ inclusion bodies are asymmetrically inherited in mammalian cell lines through the asymmetric partitioning of vimentin | journal=] | year=2014 |pmc=4050583 | volume=111 | issue=22 | pages=8049–54}}</ref> | ||
| Also, vimentin is found to control the transport of ], LDL, -derived ] from a ] to the site of esterification.<ref name="sarria">{{cite journal |vauthors=Sarria AJ, Panini SR, Evans RM | title = A functional role for vimentin intermediate filaments in the metabolism of lipoprotein-derived cholesterol in human SW-13 cells | journal = J |
Also, vimentin is found to control the transport of ], LDL, -derived ] from a ] to the site of esterification.<ref name="sarria">{{cite journal |vauthors=Sarria AJ, Panini SR, Evans RM | title = A functional role for vimentin intermediate filaments in the metabolism of lipoprotein-derived cholesterol in human SW-13 cells | journal = J Biol Chem | volume = 267 | issue = 27 | pages = 19455–63 |date=September 1992 | pmid = 1527066}}</ref> With the blocking of transport of LDL-derived cholesterol inside the cell, cells were found to store a much lower percentage of the ] than normal cells with vimentin. This dependence seems to be the first process of a biochemical function in any cell that depends on a cellular intermediate filament network. This type of dependence has ramifications on the adrenal cells, which rely on cholesteryl esters derived from LDL.<ref name="sarria" /> | ||
| Vimentin plays a role in ] formation, where it forms a cage surrounding a core of aggregated protein.<ref>{{cite journal |
Vimentin plays a role in ] formation, where it forms a cage surrounding a core of aggregated protein.<ref>{{cite journal| volume=143 | issue=7 | title=Aggresomes: a cellular response to misfolded proteins | pmc=2175217 | year=1998 | journal=J Cell Biol | pages=1883–98| vauthors=Johnston JA, Ward CL, Kopito RR}}</ref> | ||
| == Clinical significance == | == Clinical significance == | ||
| It has been used as a ] ] to identify ].<ref name="pmid2435649">{{cite journal |vauthors=Leader M, Collins M, Patel J, Henry K |title=Vimentin: an evaluation of its role as a tumour marker |journal=Histopathology |volume=11 |issue=1 |pages=63–72 |date=January 1987 |pmid=2435649 |
It has been used as a ] ] to identify ].<ref name="pmid2435649">{{cite journal |vauthors=Leader M, Collins M, Patel J, Henry K |title=Vimentin: an evaluation of its role as a tumour marker |journal=Histopathology |volume=11 |issue=1 |pages=63–72 |date=January 1987 |pmid=2435649}}</ref><ref name="urlImmunohistochemistry from the Washington Animal Disease Diagnostic laboratory (WADDL)of the College of Veterinary Medicine, Washington State University">{{cite web|url=http://www.vetmed.wsu.edu/depts_Waddl/ICS.aspx|title=Immunohistochemistry from the Washington Animal Disease Diagnostic laboratory (WADDL)of the College of Veterinary Medicine, Washington State University|format=|website=|accessdate=2009-03-14|archive-url=https://web.archive.org/web/20081201043331/http://www.vetmed.wsu.edu/depts_waddl/ICS.aspx|archive-date=2008-12-01|dead-url=yes|df=}}</ref> | ||
| Methylation of the vimentin gene has been established as a biomarker of colon cancer and this is being utilized in the development of fecal tests for colon cancer. Statistically significant levels of vimentin gene methylation have also been observed in certain upper gastrointestinal pathologies such as ], esophageal adenocarcinoma, and intestinal type gastric cancer.<ref>{{cite journal|last=Moinova|first=Helen|title=Aberrant Vimentin Methylation is Characteristic of Upper GI Pathologies|journal=Cancer Epidemiology, Biomarkers & Prevention|date=April 2012|volume=21|issue=4|pages=594–600 |
Methylation of the vimentin gene has been established as a biomarker of colon cancer and this is being utilized in the development of fecal tests for colon cancer. Statistically significant levels of vimentin gene methylation have also been observed in certain upper gastrointestinal pathologies such as ], esophageal adenocarcinoma, and intestinal type gastric cancer.<ref>{{cite journal|last=Moinova|first=Helen|title=Aberrant Vimentin Methylation is Characteristic of Upper GI Pathologies|journal=Cancer Epidemiology, Biomarkers & Prevention|date=April 2012|volume=21|issue=4|pages=594–600|pmc=3454489}}</ref> High levels of DNA methylation in the promoter region have also been associated with markedly decreased survival in hormone positive breast cancers.<ref>{{cite journal|last=Ulirsch|first=Jacob|title=Vimentin DNA methylation predicts survival in breast cancer|journal=Breast Cancer Research and Treatment|date=January 2013|pmid=23239149|volume=137|issue=2|pages=383–96|pmc=3838916}}</ref> | ||
| Downregulation of vimentin was identified in cystic variant of ] using a proteomic approach.<ref name=pmid25978681>{{cite journal | vauthors = Dinets A, Pernemalm M, Kjellin H, Sviatoha V, Sofiadis A, Juhlin CC, Zedenius J, Larsson C, Lehtiö J, Höög A | title = Differential protein expression profiles of cyst fluid from papillary thyroid carcinoma and benign thyroid lesions | journal = PLOS ONE | volume = 10 | issue = 5 | pages = e0126472 | date = May 2015 |
Downregulation of vimentin was identified in cystic variant of ] using a proteomic approach.<ref name=pmid25978681>{{cite journal | vauthors = Dinets A, Pernemalm M, Kjellin H, Sviatoha V, Sofiadis A, Juhlin CC, Zedenius J, Larsson C, Lehtiö J, Höög A | title = Differential protein expression profiles of cyst fluid from papillary thyroid carcinoma and benign thyroid lesions | journal = PLOS ONE | volume = 10 | issue = 5 | pages = e0126472 | date = May 2015| pmc=4433121}}</ref> | ||
| See also ] for its use in diagnosis of ]. | See also ] for its use in diagnosis of ]. | ||
| ==Interactions== | ==Interactions== | ||
| Vimentin has been shown to ] with: | Vimentin has been shown to ] with: | ||
| * ] <ref name = pmid9261168>{{cite journal |vauthors=Meng JJ, Bornslaeger EA, Green KJ, Steinert PM, Ip W | title = Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments | journal = J |
* ] <ref name = pmid9261168>{{cite journal |vauthors=Meng JJ, Bornslaeger EA, Green KJ, Steinert PM, Ip W | title = Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments | journal = J Biol Chem | volume = 272 | issue = 34 | pages = 21495–503 | year = 1997 | pmid = 9261168}}</ref> | ||
| * ] <ref name = pmid12169273>{{cite journal |vauthors=Lopez-Egido J, Cunningham J, Berg M, Oberg K, Bongcam-Rudloff E, Gobl A | title = Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity | journal = Exp |
* ] <ref name = pmid12169273>{{cite journal |vauthors=Lopez-Egido J, Cunningham J, Berg M, Oberg K, Bongcam-Rudloff E, Gobl A | title = Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity | journal = Exp Cell Res | volume = 278 | issue = 2 | pages = 175–83 | year = 2002 | pmid = 12169273}}</ref> | ||
| * ] <ref name = pmid16189514>{{cite journal|authorlink30=Huda Zoghbi |vauthors=Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M | title = Towards a proteome-scale map of the human protein-protein interaction network | journal = Nature | volume = 437 | issue = 7062 | pages = 1173–8 | year = 2005 | pmid = 16189514 |
* ] <ref name = pmid16189514>{{cite journal|authorlink30=Huda Zoghbi |vauthors=Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M | title = Towards a proteome-scale map of the human protein-protein interaction network | journal = Nature | volume = 437 | issue = 7062 | pages = 1173–8 | year = 2005 | pmid = 16189514}}</ref><ref name = pmid16169070>{{cite journal |vauthors=Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE | title = A human protein-protein interaction network: a resource for annotating the proteome | journal = Cell | volume = 122 | issue = 6 | pages = 957–68 | year = 2005 | pmid = 16169070}}</ref> | ||
| * ] <ref name = |
* ] <ref name = pmid9175763>{{cite journal |vauthors=Matsuzawa K, Kosako H, Inagaki N, Shibata H, Mukai H, Ono Y, Amano M, Kaibuchi K, Matsuura Y, Azuma I, Inagaki M | title = Domain-specific phosphorylation of vimentin and glial fibrillary acidic protein by PKN | journal = Biochem Biophys Res Commun | volume = 234 | issue = 3 | pages = 621–5 | year = 1997 | pmid = 9175763}}</ref> | ||
| ⚫ | * ] <ref name = pmid29048609>{{cite journal |vauthors=Ratnayake WS, Apostolatos AH, Ostrov DA, Acevedo-Duncan M | title = Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis | journal = Int J Oncol | volume = 51 | issue = 5 | pages = 1370–1382 | year = 2017| pmc=5642393}}</ref><ref name = pmid29781749>{{cite journal |vauthors=Ratnayake WS, Apostolatos CA, Apostolatos AH, Schutte RJ, Huynh MA, Ostrov DA, Acevedo-Duncan M | title = Oncogenic PKC-ι activates Vimentin during epithelial-mesenchymal transition in melanoma; a study based on PKC-ι and PKC-ζ specific inhibitors | journal = Cell Adhes Migr| volume = 0 | pages = 1–17 | year = 2018| pmc = 6363030}}</ref> | ||
| * ] <ref name = pmid9175763>{{cite journal |vauthors=Matsuzawa K, Kosako H, Inagaki N, Shibata H, Mukai H, Ono Y, Amano M, Kaibuchi K, Matsuura Y, Azuma I, Inagaki M | title = Domain-specific phosphorylation of vimentin and glial fibrillary acidic protein by PKN | journal = Biochem. Biophys. Res. Commun. | volume = 234 | issue = 3 | pages = 621–5 | year = 1997 | pmid = 9175763 | doi = 10.1006/bbrc.1997.6669 }}</ref> | |||
| * ] <ref name = pmid3027087>{{cite journal |vauthors=Herrmann H, Wiche G | title = Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin | journal = J Biol Chem | volume = 262 | issue = 3 | pages = 1320–5 | year = 1987 | pmid = 3027087}}</ref><ref name = pmid11441066>{{cite journal |vauthors=Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S | title = Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin | journal = J Immunol | volume = 167 | issue = 2 | pages = 641–5 | year = 2001 | pmid = 11441066}}</ref> | |||
| * ] <ref name = pmid11441066/> | * ] <ref name = pmid11441066/> | ||
| * ] <ref name = pmid11278417>{{cite journal |vauthors=Russell RL, Cao D, Zhang D, Handschumacher RE, Pizzorno G | title = Uridine phosphorylase association with vimentin. Intracellular distribution and localization | journal = J |
* ] <ref name = pmid11278417>{{cite journal |vauthors=Russell RL, Cao D, Zhang D, Handschumacher RE, Pizzorno G | title = Uridine phosphorylase association with vimentin. Intracellular distribution and localization | journal = J Biol Chem | volume = 276 | issue = 16 | pages = 13302–7 | year = 2001 | pmid = 11278417}}</ref> | ||
| * ] <ref name = pmid10887173>{{cite journal |vauthors=Tzivion G, Luo ZJ, Avruch J | title = Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo | journal = J |
* ] <ref name = pmid10887173>{{cite journal |vauthors=Tzivion G, Luo ZJ, Avruch J | title = Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo | journal = J Biol Chem | volume = 275 | issue = 38 | pages = 29772–8 | year = 2000 | pmid = 10887173}}</ref> | ||
| {{Div col end}} | |||
| ⚫ | * ] <ref name = pmid29048609>{{cite journal |vauthors=Ratnayake WS, Apostolatos AH, Ostrov DA, Acevedo-Duncan M | title = Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis | journal = Int |
||
| {{Div col end}}<ref name = pmid29781749>{{cite journal |vauthors=Ratnayake WS, Apostolatos CA, Apostolatos AH, Schutte RJ, Huynh MA, Ostrov DA, Acevedo-Duncan M | title = Oncogenic PKC-ι activates Vimentin during epithelial-mesenchymal transition in melanoma; a study based on PKC-ι and PKC-ζ specific inhibitors | journal = Cell Adhes. Migr.| volume = 0 | pages = 1–17 | year = 2018 | pmid = 29781749 | pmc = 6363030 | doi = 10.1080/19336918.2018.1471323}}</ref> | |||
| {{Div col end}} | |||
| ⚫ | The ] has been found to bind a 46kDa protein.<ref name="pmid9241253">{{cite journal |vauthors=Zehner ZE, Shepherd RK, Gabryszuk J, Fu TF, Al-Ali M, Holmes WM | title = RNA-protein interactions within the 3 ' untranslated region of vimentin mRNA | journal = Nucleic Acids Res. | volume = 25 | issue = 16 | pages = 3362–70 |date=August 1997| pmc = 146884}}</ref> | ||
| ⚫ | The ] has been found to bind a 46kDa protein.<ref name="pmid9241253">{{cite journal |vauthors=Zehner ZE, Shepherd RK, Gabryszuk J, Fu TF, Al-Ali M, Holmes WM | title = RNA-protein interactions within the 3 ' untranslated region of vimentin mRNA | journal = Nucleic Acids Res. | volume = 25 | issue = 16 | pages = 3362–70 |date=August 1997 |
||
| == References == | == References == | ||
| Line 57: | Line 53: | ||
| == Further reading == | == Further reading == | ||
| {{Refbegin | 2}} | {{Refbegin | 2}} | ||
| *{{cite journal |
*{{cite journal |vauthors=Snásel J, Pichová I |title=The cleavage of host cell proteins by HIV-1 protease |journal=Folia Biol. (Praha) |volume=42 |issue= 5 |pages= 227–30 |year= 1997 |pmid= 8997639}} | ||
| *{{cite journal |
*{{cite journal |vauthors=Lake JA, Carr J, Feng F, etal |title=The role of Vif during HIV-1 infection: interaction with novel host cellular factors |journal=J Clin Virol |volume=26 |issue= 2 |pages= 143–52 |year= 2003 |pmid= 12600646}} | ||
| {{Refend}} | {{Refend}} | ||
Revision as of 19:08, 25 July 2019
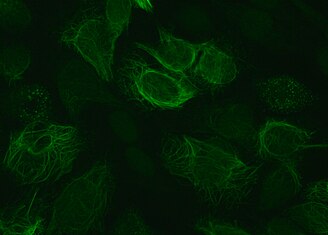
Vimentin is a structural protein that in humans is encoded by the VIM gene. Its name comes from the Latin vimentum which refers to an array of flexible rods.

Vimentin is a type III intermediate filament (IF) protein that is expressed in mesenchymal cells. IF proteins are found in all animal cells as well as bacteria. IF, along with tubulin-based microtubules and actin-based microfilaments, comprises the cytoskeleton. All IF proteins are expressed in a highly developmentally-regulated fashion; vimentin is the major cytoskeletal component of mesenchymal cells. Because of this, vimentin is often used as a marker of mesenchymally-derived cells or cells undergoing an epithelial-to-mesenchymal transition (EMT) during both normal development and metastatic progression.
Structure
A vimentin monomer, like all other intermediate filaments, has a central α-helical domain, capped on each end by non-helical amino (head) and carboxyl (tail) domains. Two monomers are likely co-translationally expressed in a way that facilitates their formation of a coiled-coil dimer, which is the basic subunit of vimentin assembly.
The α-helical sequences contain a pattern of hydrophobic amino acids that contribute to forming a "hydrophobic seal" on the surface of the helix. In addition, there is a periodic distribution of acidic and basic amino acids that seems to play an important role in stabilizing coiled-coil dimers. The spacing of the charged residues is optimal for ionic salt bridges, which allows for the stabilization of the α-helix structure. While this type of stabilization is intuitive for intrachain interactions, rather than interchain interactions, scientists have proposed that perhaps the switch from intrachain salt bridges formed by acidic and basic residues to the interchain ionic associations contributes to the assembly of the filament.
Function
Vimentin plays a significant role in supporting and anchoring the position of the organelles in the cytosol. Vimentin is attached to the nucleus, endoplasmic reticulum, and mitochondria, either laterally or terminally.
The dynamic nature of vimentin is important when offering flexibility to the cell. Scientists found that vimentin provided cells with a resilience absent from the microtubule or actin filament networks, when under mechanical stress in vivo. Therefore, in general, it is accepted that vimentin is the cytoskeletal component responsible for maintaining cell integrity. (It was found that cells without vimentin are extremely delicate when disturbed with a micropuncture). Transgenic mice that lack vimentin appeared normal and did not show functional differences. It is possible that the microtubule network may have compensated for the absence of the intermediate network. This result supports an intimate interactions between microtubules and vimentin. Moreover, when microtubule depolymerizers were present, vimentin reorganization occurred, once again implying a relationship between the two systems. On the other hand, wounded mice that lack the vimentin gene heal slower than their wild type counterparts.
In essence, vimentin is responsible for maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions. Vimentin has been shown to eliminate toxic proteins in JUNQ and IPOD inclusion bodies in asymmetric division of mammalian cell lines.
Also, vimentin is found to control the transport of low-density lipoprotein, LDL, -derived cholesterol from a lysosome to the site of esterification. With the blocking of transport of LDL-derived cholesterol inside the cell, cells were found to store a much lower percentage of the lipoprotein than normal cells with vimentin. This dependence seems to be the first process of a biochemical function in any cell that depends on a cellular intermediate filament network. This type of dependence has ramifications on the adrenal cells, which rely on cholesteryl esters derived from LDL.
Vimentin plays a role in aggresome formation, where it forms a cage surrounding a core of aggregated protein.
Clinical significance
It has been used as a sarcoma tumor marker to identify mesenchyme.
Methylation of the vimentin gene has been established as a biomarker of colon cancer and this is being utilized in the development of fecal tests for colon cancer. Statistically significant levels of vimentin gene methylation have also been observed in certain upper gastrointestinal pathologies such as Barrett's esophagus, esophageal adenocarcinoma, and intestinal type gastric cancer. High levels of DNA methylation in the promoter region have also been associated with markedly decreased survival in hormone positive breast cancers. Downregulation of vimentin was identified in cystic variant of papillary thyroid carcinoma using a proteomic approach. See also Anti-citrullinated protein antibody for its use in diagnosis of rheumatoid arthritis.
Interactions
Vimentin has been shown to interact with:
The 3' UTR of Vimentin mRNA has been found to bind a 46kDa protein.
References
- ^ GRCh38: Ensembl release 89: ENSG00000026025 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000026728 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Franke, WW; Schmid, E; Osborn, M; Weber, K (October 1978). "Different intermediate-sized filaments distinguished by immunofluorescence microscopy". Proc Natl Acad Sci U S A. 75 (10): 5034–8. PMC 336257.
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD (2009). "Introducing intermediate filaments: from discovery to disease". J Clin Invest. 119 (7): 1763–71. PMC 2701876.
- Cabeen MT, Jacobs-Wagner C (2010). "The bacterial cytoskeleton". Annu Rev Genet. 44: 365–92. PMID 21047262.
- ^ Fuchs E.; Weber K. (1994). "Intermediate filaments: structure, dynamics, function, and disease". Annu Rev Biochem. 63: 345–82. PMID 7979242.
- Chang L, Shav-Tal Y, Trcek T, Singer RH, Goldman RD (2006). "Assembling an intermediate filament network by dynamic cotranslation". J Cell Biol. 172 (5): 747–58. PMC 2063706.
- Katsumoto T.; Mitsushima A.; Kurimura T. (1990). "The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction". Biol Cell. 68 (2): 139–46. PMID 2192768.
- ^ Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM (August 1996). "The function of intermediate filaments in cell shape and cytoskeletal integrity". J Cell Biol. 134 (4): 971–83. PMC 2120965.
- Golucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C (1994). "Mice lacking vimentin develop and reproduce without an obvious phenotype". Cell. 79 (4): 679–94. PMID 7954832.
- Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P (2000). "Impaired wound healing in embryonic and adult mice lacking vimentin". J Cell Sci. 113: 2455–62. PMID 10852824.
- Ogrodnik M, Salmonowicz H, Brown R, Turkowska J, Sredniawa W, Pattabiraman S, Amen T, Abraham AC, Eichler N, Lyakhovetsky R, Kaganovich D (2014). "Dynamic JUNQ inclusion bodies are asymmetrically inherited in mammalian cell lines through the asymmetric partitioning of vimentin". Proc Natl Acad Sci U S A. 111 (22): 8049–54. PMC 4050583.
- ^ Sarria AJ, Panini SR, Evans RM (September 1992). "A functional role for vimentin intermediate filaments in the metabolism of lipoprotein-derived cholesterol in human SW-13 cells". J Biol Chem. 267 (27): 19455–63. PMID 1527066.
- Johnston JA, Ward CL, Kopito RR (1998). "Aggresomes: a cellular response to misfolded proteins". J Cell Biol. 143 (7): 1883–98. PMC 2175217.
- Leader M, Collins M, Patel J, Henry K (January 1987). "Vimentin: an evaluation of its role as a tumour marker". Histopathology. 11 (1): 63–72. PMID 2435649.
- "Immunohistochemistry from the Washington Animal Disease Diagnostic laboratory (WADDL)of the College of Veterinary Medicine, Washington State University". Archived from the original on 2008-12-01. Retrieved 2009-03-14.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - Moinova, Helen (April 2012). "Aberrant Vimentin Methylation is Characteristic of Upper GI Pathologies". Cancer Epidemiology, Biomarkers & Prevention. 21 (4): 594–600. PMC 3454489.
- Ulirsch, Jacob (January 2013). "Vimentin DNA methylation predicts survival in breast cancer". Breast Cancer Research and Treatment. 137 (2): 383–96. PMC 3838916. PMID 23239149.
- Dinets A, Pernemalm M, Kjellin H, Sviatoha V, Sofiadis A, Juhlin CC, Zedenius J, Larsson C, Lehtiö J, Höög A (May 2015). "Differential protein expression profiles of cyst fluid from papillary thyroid carcinoma and benign thyroid lesions". PLOS ONE. 10 (5): e0126472. PMC 4433121.
- Meng JJ, Bornslaeger EA, Green KJ, Steinert PM, Ip W (1997). "Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments". J Biol Chem. 272 (34): 21495–503. PMID 9261168.
- Lopez-Egido J, Cunningham J, Berg M, Oberg K, Bongcam-Rudloff E, Gobl A (2002). "Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity". Exp Cell Res. 278 (2): 175–83. PMID 12169273.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. PMID 16189514.
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. PMID 16169070.
- Matsuzawa K, Kosako H, Inagaki N, Shibata H, Mukai H, Ono Y, Amano M, Kaibuchi K, Matsuura Y, Azuma I, Inagaki M (1997). "Domain-specific phosphorylation of vimentin and glial fibrillary acidic protein by PKN". Biochem Biophys Res Commun. 234 (3): 621–5. PMID 9175763.
- Ratnayake WS, Apostolatos AH, Ostrov DA, Acevedo-Duncan M (2017). "Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis". Int J Oncol. 51 (5): 1370–1382. PMC 5642393.
- Ratnayake WS, Apostolatos CA, Apostolatos AH, Schutte RJ, Huynh MA, Ostrov DA, Acevedo-Duncan M (2018). "Oncogenic PKC-ι activates Vimentin during epithelial-mesenchymal transition in melanoma; a study based on PKC-ι and PKC-ζ specific inhibitors". Cell Adhes Migr. 0: 1–17. PMC 6363030.
- Herrmann H, Wiche G (1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". J Biol Chem. 262 (3): 1320–5. PMID 3027087.
- ^ Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S (2001). "Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin". J Immunol. 167 (2): 641–5. PMID 11441066.
- Russell RL, Cao D, Zhang D, Handschumacher RE, Pizzorno G (2001). "Uridine phosphorylase association with vimentin. Intracellular distribution and localization". J Biol Chem. 276 (16): 13302–7. PMID 11278417.
- Tzivion G, Luo ZJ, Avruch J (2000). "Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo". J Biol Chem. 275 (38): 29772–8. PMID 10887173.
- Zehner ZE, Shepherd RK, Gabryszuk J, Fu TF, Al-Ali M, Holmes WM (August 1997). "RNA-protein interactions within the 3 ' untranslated region of vimentin mRNA". Nucleic Acids Res. 25 (16): 3362–70. PMC 146884.
Further reading
- Snásel J, Pichová I (1997). "The cleavage of host cell proteins by HIV-1 protease". Folia Biol. (Praha). 42 (5): 227–30. PMID 8997639.
- Lake JA, Carr J, Feng F, et al. (2003). "The role of Vif during HIV-1 infection: interaction with novel host cellular factors". J Clin Virol. 26 (2): 143–52. PMID 12600646.
External links
| PDB gallery | |
|---|---|
| Proteins of the cytoskeleton | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nonhuman | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| See also: cytoskeletal defects | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tumor markers | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood |
| ||||||||||||
| Endocrine |
| ||||||||||||
| Nervous system |
| ||||||||||||
| Cardiovascular/ respiratory |
| ||||||||||||
| Digestive |
| ||||||||||||
| Reproductive/ urinary/ breast |
| ||||||||||||
| General histology |
| ||||||||||||
| Musculoskeletal |
| ||||||||||||